Main Article Content
Abstract
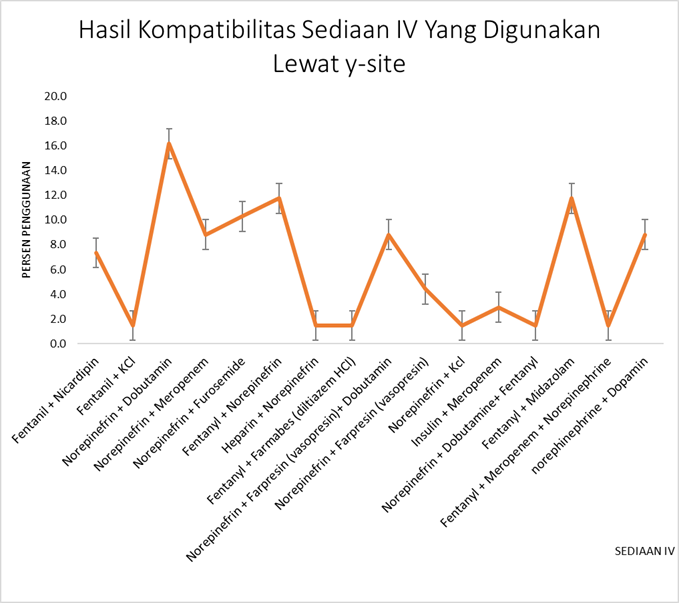
The simultaneous use of intravenous (IV) preparations through Y-site administration or the same catheter line can lead to changes in product compatibility. This study aims to evaluate the compatibility of IV preparation mixtures using a comprehensive database. A cross-sectional observational method was employed, with data collected prospectively as researchers directly observed drug administration during the study. A total of 100 IV dosage mixtures were obtained from 37 patients between June and September 2023. The data were analyzed using the online Lexicomp database and the Handbook on Injectable Drugs in 2017 by American Society of Health-System Pharmacists (ASHP). The compatibility assessment revealed that 68% of the IV mixtures were compatible (C), 19% were incompatible (I), and 13% had no available information (NI). These findings underscore the importance of careful consideration of potential incompatibilities during concomitant drug administration.
Article Details

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.